MOVERS, SHAKERS
& PATHBREAKERS
Company
Sector
Location
Ansella Therapeutics
Specialty Pharma
US

Ansella Therapeutics is specialty pharmaceutical company that leverages biomimetic sciences to develop novel therapeutics that improve healthcare outcomes. The company’s initial focus is on women’s health, specifically in the development of novel vaginal formulations.

Emulating Biology to Make Medicine Better
Company Overview
Ansella Therapeutics is specialty pharmaceutical company that leverages biomimetic sciences to develop novel therapeutics that improve healthcare outcomes. The company’s initial focus is on women’s health, specifically in the development of novel vaginal formulations.
Biomimetics is an interdisciplinary field in which principles from engineering, chemistry and biology are applied to the synthesis of materials, or synthetic systems that have functions that mimic biological processes. Biomaterials are any natural or synthetic material that interacts with any part of a biological system. Biomimetic designs can be used in regenerative medicine, tissue engineering and drug delivery.
Ansella Therapeutics utilizes its expertise in materials science and biomedical engineering to create unique, enhanced therapeutic approaches inspired by inherent anatomy, physiology, biochemistry, and endocrinology.
The company has launched its first product, Cerynë Intimate Care, for women with everyday vaginal dryness and designed for vulvovaginal application. Cerynë Intimate Care is the world’s first biomimetic formulation which works with your body to restore comfort and balance.
Cerynë Intimate care has been launched both directly to consumers and through physician channels as an intimate care product for vaginal dryness.
Ansella Therapeutics is developing a range of treatment that focus on Women’s Health including Menopause, Vaginal mucositis, Endometriosis, Uterine Fibroids and vaginal Infection.
Ansella Therapeutics is specialty pharmaceutical company that leverages biomimetic sciences to develop novel therapeutics that improve healthcare outcomes. The company’s initial focus is on women’s health, specifically in the development of novel vaginal formulations.
Avive Solutions
Medical Device
US

Avive is a MedTech company that has developed a next-generation automated external defibrillator (AED) and software solutions that revolutionizes the current sudden cardiac arrest (SCA) response.

Transforming the sudden cardiac arrest response
Company overview
Avive Solutions is a MedTech company that has developed a next-generation automated external defibrillator (AED) and software solutions that revolutionizes the current sudden cardiac arrest (SCA) response.
The device aims to significantly increase survival rates from SCA.
The $3.5 billion global external defibrillators market is projected to have 9.7% compounded annual growth rate (CAGR) from 2024 to 2030. The AED market growth is driven by legislation, property development and hospital groups, which has caused a shift in customer awareness and push for inbound sales models that the large players generally ignore in favor of entrenched distributor relationships.
The current SCA response system has a 10% survival rate, but when AEDs are used, the survival rate increases to over 40%. The low AED usage is due to limited availability, improper maintenance, and a lack of connectivity in current AED machines.
Avive’s automated external defibrillator (AED) device is a next-generation IoT (internet-of-things) device with complementary software solutions that has the ability to transform the existing flawed system for responding to Sudden Cardiac Arrest (SCA). Their system automates AED maintenance, tracks the AED, provides remote software updates, and automatically shares rescue data with emergency services, effectively eliminating the issues plaguing current aed machines. Their goal is to get an AED to a patient in under 4 minutes compared to the normal standard response time for emergency services which is 12 minutes.
Avive is a MedTech company that has developed a next-generation automated external defibrillator (AED) and software solutions that revolutionizes the current sudden cardiac arrest (SCA) response.
Caresyntax
HEALTHTECH
US

Caresyntax offers AI-powered software, devices, and clinical services designed to enhance surgical outcomes. With a significant global presence in operating rooms, their technology has been utilized in millions of surgeries and boasts a substantial user base of surgical professionals.

Using AI to Make Surgery Safer and Smarter
Company Overview
Caresyntax has developed an enterprise-grade surgical intelligence system that is vendor-neutral and equipped with proprietary software, hardware, and advanced AI capabilities. It can ingest and analyze data throughout the surgical workflow, including EMR, video, audio, image, device data, financial and outcomes data, and clinical and operational metrics to capture a complete picture of the surgical pathway. This allows the platform to generate insights across the entire continuum of care—before the procedure with surgical workflow optimization, during surgery with real-time clinically relevant support tools, and post procedure with clinical safety modules. The platform also produces data analytics and insights for medical device companies, insurers, and other ecosystem players to innovate and drive the future of healthcare delivery.
The Company has a footprint in 3,000 operating rooms, has been used in over 3 million surgeries, and has over 30,000 surgical users.
Caresyntax offers AI-powered software, devices, and clinical services designed to enhance surgical outcomes. With a significant global presence in operating rooms, their technology has been utilized in millions of surgeries and boasts a substantial user base of surgical professionals.
Endeavor Biomedicines
Biotech
US

Endeavor’s ENV-101 is a second-generation Hedgehog inhibitor with a highly favorable efficacy and side-effect profile compared to the molecules currently in the market. ENV-101 inhibits target pathway by ~90% at all tested doses.

Targeted treatments for fibrosis and cancer
Company overview
Edeavors ENV-101 is a second-generation Hedgehog inhibitor with a highly favorable efficacy and side-effect profile compared to the molecules currently in the market. ENV-101 inhibits target pathway by ~90% at all tested doses.
Currently Hedgehog inhibitors are indicated for basal cell carcinoma. Significant data indicates the pathway involvement in fibrosis of the lungs. ENV-101 represents a significant improvement on existing therapies for IPF and is expected to be the Best-in-Class Hedgehog Inhibitor.
Attractive white space in the market
IPF market estimated to be $8.8B by 2027. SOC (standard of care), i.e., Boehringer Ingelheim’s Nintedanib (Ofev) has current sales >$3B with <20% market penetration based on prescription data. Current SOC has poor tolerability and efficacy.
Roche acquired InterMune in 2014 for $8.3 billion for Pirfenidone (Esbriet), indicated for IPF. Esbriet lost exclusivity in 2022 and Ofev is expected to lose exclusivity in 2025; which gives the next blockbuster significant white space for pricing and reimbursement. Both Ofev and Esbriet have severe and limiting GI issues for patients which ENV-101 seems to address.
Endeavor’s ENV-101 is a second-generation Hedgehog inhibitor with a highly favorable efficacy and side-effect profile compared to the molecules currently in the market. ENV-101 inhibits target pathway by ~90% at all tested doses.
Enquyst Technologies
Biotech Services
US

Enquyst Technologies is developing the next-generation biopharmaceutical manufacturing platform which aims to redesign the downstream bioprocess equipment to bring unprecedented advantages in cost, time, footprint, and speed to market at commercial scale.
Lowering the cost of biologic manufacturing
Company overview
Enquyst Technologies is developing the next-generation biopharmaceutical manufacturing platform which aims to redesign the downstream bioprocess equipment to bring unprecedented advantages in cost, time, footprint, and speed to market at commercial scale.
The technology aims to be the industry standard for downstream processing.
Disrupt the business model and expand markets in the $400 billion biologics market
The current downstream processing for expensive biologics, gene therapy, and viral vectors is expensive, cumbersome, and is fraught with regulatory challenges across the manufacturing spectrum.
Additionally, these expensive therapeutics are generally out of reach for most emerging countries. Enquyst plans to democratize the manufacturing process by bringing next-generation manufacturing that is fully continuous, reduces capital expenditure and operating expenditure by over 70%, reduces the footprint of manufacturing by over 70% and can reduce the time to setup a new facility from the current 5-years to under 3-years.
The technology can be game-changer for gene-therapy manufacturers, biosimilar companies, as well as early-stage biotech companies where product purification, separation, and product consistency can be the difference between success and failure.
CSO: Dr. DEE atwal

Dee has over 3 decades of experience in antibody engineering and development, covering multiple antibody programs now on the market. Dee has managed and led programs spanning oncology, autoimmune, inflammatory and CNS from discovery through clinical development.
founder: Dr. Jason Criscione

Dr. Criscione is an applied science and engineering entrepreneur with a unique expertise that covers a variety of different scientific disciplines. Dr. Criscione has 9+ years of experience in successfully building and leading interdisciplinary technical teams for start-up biotech and pharmaceutical companies. Dr. Criscione has published in numerous peer-reviewed, scientific journals, including, Nature Nanotechnology, Nature Materials, Biomaterials, JACS, ACS Nano, and Bioconjugate Chemistry. Dr. Criscione received his Ph.D. in Biomedical Engineering, his Mphil in Biomedical Engineering and a MS in Biomedical engineering from Yale University. He also received a MS in Physical Chemistry from Michigan State University and a BA with High Honors in Chemistry and a concentration in Neuroscience from Oberlin College.
Enquyst Technologies is developing the next-generation biopharmaceutical manufacturing platform which aims to redesign the downstream bioprocess equipment to bring unprecedented advantages in cost, time, footprint, and speed to market at commercial scale.
FLUID AI
Healtech
Canada

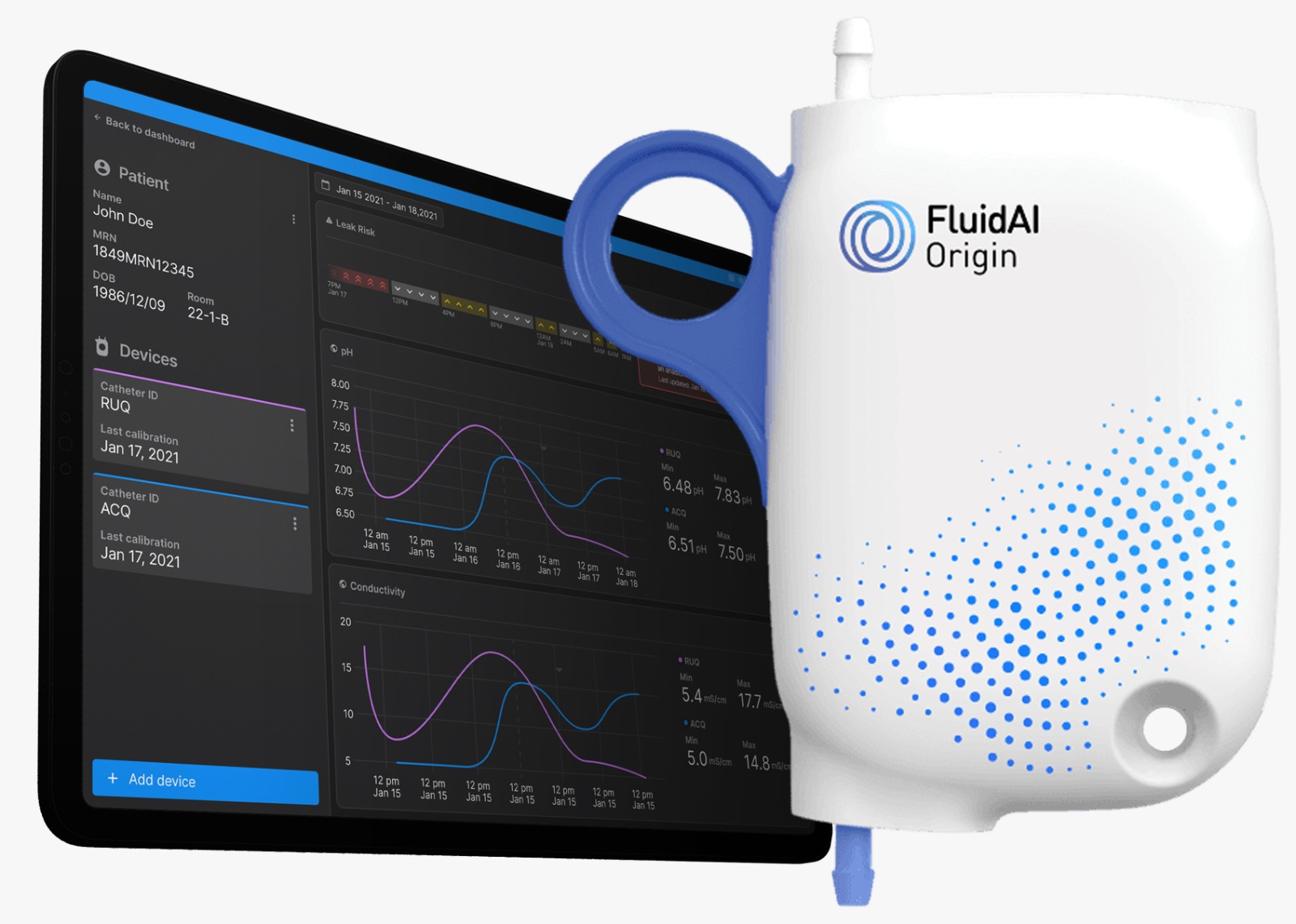
FluidAI is a healthcare technology company developing artificial intelligence–driven solutions to improve the management of fluid status in hospitalized patients. The company’s platform is designed to support earlier clinical decision making and reduce complications associated with fluid imbalance, a key driver of morbidity, length of stay, and healthcare costs.
Company Overview
FluidAI is a healthcare technology company developing artificial intelligence–driven solutions to improve the management of fluid status in hospitalized patients. The company’s platform is designed to support earlier clinical decision making and reduce complications associated with fluid imbalance, a key driver of morbidity, length of stay, and healthcare costs.
FluidAI’s technology leverages continuous physiological data and advanced machine learning models to generate actionable insights for clinicians, enabling proactive intervention before adverse events occur. By integrating seamlessly into existing clinical workflows, the platform aims to enhance patient safety while improving efficiency in acute and critical care settings.
Addressing a large, global inpatient market with significant unmet need, FluidAI is positioned to benefit from growing demand for data-driven, preventative care solutions. The company’s approach aligns with health system priorities around outcome improvement, cost containment, and the adoption of clinically validated AI technologies.
FluidAI is a healthcare technology company developing artificial intelligence–driven solutions to improve the management of fluid status in hospitalized patients. The company’s platform is designed to support earlier clinical decision making and reduce complications associated with fluid imbalance, a key driver of morbidity, length of stay, and healthcare costs.
Huma Therapeutics
HEALTHTECH
UK
Huma is a global healthcare AI company on a mission to accelerate the adoption of digital solutions in care and research. The company is renowned for its role in major national healthcare projects worldwide as well as collaborating with most large pharma companies

DIGITAL SOLUTIONS IN CARE AND RESEARCH
Company Overview
Huma Therapeutics is revolutionizing healthcare by enabling remote patient monitoring and decentralized clinical trials through its advanced, condition-agnostic platforms. Huma’s mission is to shift healthcare from hospital-centric to home-centric, enabling proactive and personalized care. Its award-winning modular platforms are used by more than 3,000 hospitals and clinics, with over 35 million screened users and 4 million registered users in healthcare and has powered over 800 studies supporting about 1 million participants across research.
The Company is in high growth mode and actively pursuing both M&A and IPO options but isn’t looking to commit to either option at this point. The management believes that they have significant growth potential and will choose the appropriate exit path when it is optimum for their shareholders.
Huma is a global healthcare AI company on a mission to accelerate the adoption of digital solutions in care and research. The company is renowned for its role in major national healthcare projects worldwide as well as collaborating with most large pharma companies
KARDIUM
Medtech
Canada

Kardium is a medical technology company focused on the treatment of atrial fibrillation through advanced cardiac mapping and ablation solutions. The company has developed a differentiated platform designed to improve procedural efficiency, clinical outcomes, and physician workflow in the electrophysiology lab.
Company Overview
Kardium is a medical technology company focused on the treatment of atrial fibrillation through advanced cardiac mapping and ablation solutions. The company has developed a differentiated platform designed to improve procedural efficiency, clinical outcomes, and physician workflow in the electrophysiology lab.
Kardium’s proprietary technology integrates high-resolution mapping with multi- electrode ablation in a single system, enabling more precise identification and treatment of complex arrhythmias. The platform is designed to address limitations of conventional ablation approaches by reducing procedure time and increasing consistency, while maintaining a strong focus on safety and clinical performance.
With regulatory progress and growing clinical adoption, Kardium is positioned to play a meaningful role in the evolving electrophysiology market. The company addresses a large and expanding global patient population, supported by favorable demographic trends and increasing demand for minimally invasive cardiac interventions.
Kardium is a medical technology company focused on the treatment of atrial fibrillation through advanced cardiac mapping and ablation solutions. The company has developed a differentiated platform designed to improve procedural efficiency, clinical outcomes, and physician workflow in the electrophysiology lab.
NETTWORK HOLDINGS
distribution
US

Nettwork Holdings is a distribution focused company that supports commercialization efforts across the healthcare ecosystem.
OPENING COMMERCIAL OPPORTUNITIES ACROSS HEALTHCARE
Company Overview
Nettwork Holdings is a distribution and marketing services company, that enables effective and targeted distribution of multiple products across the life science and healthcare value chain.
We believe that an effective distribution strategy is paramount in the current healthcare environment, and for all young companies across devices, diagnostics, digital health, nutrition and wellness, having a defined distribution strategy is critical to success.
Through a powerful distributor network, we provide access to new markets for cutting-edge medical and diagnostics products in US, Europe, Africa, and Asia.
Our partners include large hospital networks, major suppliers and distributors of healthcare products and services, government organizations, and non-profit organizations.
Our distribution services include marketing, branding, sales and support with both clinical trial strategy and design and regulatory strategy.
Nettwork Holding’s unique marketing approach enables precise measurement of factors that lead to marketing success, higher profitability, and sustained margins. The Company emphasizes Econometric Models as opposed to traditional Attribution Models that often provide inaccurate results.
Nettwork Holdings is a distribution focused company that supports commercialization efforts across the healthcare ecosystem.
PTO
Health & Wellness
US

An athlete-owned entity that supports non-drafting professional triathletes, the PTO is unique as a professional sports organisation in representing both pro women and men and through our athletes being self-determined co-owners.

Building an infrastructure to support triathletes
Company overview
An athlete-owned entity that supports non-drafting professional triathletes, the PTO is unique as a professional sports organisation in representing both pro women and men and through our athletes being self-determined co-owners
The organisation aims to be the infrastructure enabling the main streaming and monetization of triathlon.
The current triathlon Events primarily target mass amateur participants and operate on low production budgets, making them less appealing to broadcasters even with the high participation rates of around 35M athletes that triathlon achieves. However, PTO has forged partnerships with NBC, achieving viewership that surpasses established sports like PGA and MLS.
The value lies in the ability to deliver a highly engaged audience to sponsors and advertisers. Triathlon has the sport’s most valuable audience – highest HHI of any sporting audience – with 44% of triathletes who consider it into financial decisions made at their place of business.
Additionally, the triathlon audience is known to be elusive and difficult to reach, prompting advertisers to pay a premium to reach. Similar to the broader sports industry, revenues in triathlon come from sponsorship, broadcast rights, and fees from host cities for major events.
This organization can be a game changer for the triathlon industry through them building an infrastructure that enables the broadcast, sponsorship, and hosting of professional triathlon events all across the globe.
An athlete-owned entity that supports non-drafting professional triathletes, the PTO is unique as a professional sports organisation in representing both pro women and men and through our athletes being self-determined co-owners.
Samsara Vision
Med Device
US

Samsara Vision is a medical device company focused on advancing treatments for serious retinal diseases through innovative ophthalmic implant technologies. The company is developing solutions designed to address significant unmet needs in patients with advanced retinal degeneration, where existing therapeutic options are limited.
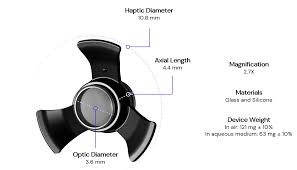
Company Overview
Samsara Vision is a medical device company focused on advancing treatments for serious retinal diseases through innovative ophthalmic implant technologies. The company is developing solutions designed to address significant unmet needs in patients with advanced retinal degeneration, where existing therapeutic options are limited.
Samsara Vision’s platform centers on a surgically implanted device intended to restore or preserve functional vision by enabling controlled drug delivery and long-term therapeutic intervention at the retinal level. The company’s approach emphasizes durability, precision, and compatibility with established ophthalmic surgical techniques, supporting both clinical adoption and patient outcomes.
Targeting a growing global population affected by degenerative retinal conditions, Samsara Vision operates at the intersection of medical need, technological innovation, and favorable demographic trends. With continued clinical development and regulatory progress, the company is positioned to play a meaningful role in the evolving retinal therapeutics and ophthalmic device market.
Samsara Vision is a medical device company focused on advancing treatments for serious retinal diseases through innovative ophthalmic implant technologies. The company is developing solutions designed to address significant unmet needs in patients with advanced retinal degeneration, where existing therapeutic options are limited.
Sanoculis
Med Device
Israel

Sanoculis has developed MIMS (Minimally Invasive Micro Sclerostomy), for treating glaucoma – the only minimally invasive procedure that delivers excellent reductions in intra-ocular pressure but leaves no implant in the eye. A MIMS procedure can be performed in about 5 minutes and requires significantly less operator skill, this means lower skilled operators can treat a higher volume of patients.

Stentless interventional galucoma surgery
Company overview
Sanoculis has developed MIMS (Minimally Invasive Micro Sclerostomy), for treating glaucoma – the only minimally invasive procedure that delivers excellent reductions in intra-ocular pressure but leaves no implant in the eye. A MIMS procedure can be performed in about 5 minutes and requires significantly less operator skill, this means lower skilled operators can treat a higher volume of patients. The company is currently partnering with industry leader, Bausch & Lomb.
85% Medication reduction at 12 mo.
Statistically equivalent or superior reduction of IOP & medication versus all methods
Leaves no artificial materials or implants behind
Reduced side effects, complications, & tissue disruption and less complicated revisions
Fast learning curve/adoption ease for ophthalmic surgeons

Glaucoma drainage devices held the largest market share since 2021 and have been growing at the fastest CAGR in the category. A growing geriatric population and a high prevalence of Glaucoma is fueling growth in the surgical devices market.
Sanoculis has developed MIMS (Minimally Invasive Micro Sclerostomy), for treating glaucoma – the only minimally invasive procedure that delivers excellent reductions in intra-ocular pressure but leaves no implant in the eye. A MIMS procedure can be performed in about 5 minutes and requires significantly less operator skill, this means lower skilled operators can treat a higher volume of patients.
Sonrai Analytics
Healthtech
UK

Cutting-edge technology to enable discovery within precision healthcare. Sonrai’s ability to manage and analyze integrated data uniquely positions the company to support biotech and pharma in the search for new drug development.
Unlocking critical information within big data
Company overview
Sonrai Analytics is developing cutting-edge technology to enable discovery within precision healthcare. Sonrai’s ability to manage and analyze integrated data uniquely positions the company to support biotech and pharma in the search for new drug development.
The Platform aims to elevate client data to expedite the R&d process for life science organisations.
Bioinformatics and data management is a large fast-growing market.
Enhancing efficiency in biopharma presents a substantial opportunity due to the exponential growth of scientific experiment datasets. Many biologists lack computational tools for rapid analysis, while research tracking in paper and spreadsheets hinders collaboration and reproduction.
Biopharmaceutical industry professionals have revealed challenges with Omics data, emphasizing issues with quality, storage, FAIR compliance, integration, and analysis. An estimated 70-80% of biopharmaceutical environments lack FAIR compliance. studies suggests potential savings of $9-12 million for emerging BioPharma through a 40% reduction in data wrangling, leading to increased efficiency and improved scientific outcomes.
Sonrai’s capacity to consolidate diverse datasets within a unified platform, coupled with the strength of its robust and scalable infrastructure allows for their platform to effectively manage various data modalities, enabling applications in a variety of computational inputs allowing for novel correlations. The platforms able to combine and derive insights from multiple types of data, employing AI/ML and deep learning techniques to clean and integrate large multi-omics datasets in seconds.
Cutting-edge technology to enable discovery within precision healthcare. Sonrai’s ability to manage and analyze integrated data uniquely positions the company to support biotech and pharma in the search for new drug development.
VIVANCE Technologies
Medtech
Singapore

VIVANCE Technologies is a clinical-stage, medical device company that has developed a disruptive peritoneal dialysis (PD) device based on revolutionary new filtration technology developed in-house.
Bringing a paradigm shift in kidney care
Company overview
VIVANCE Technologies is a clinical-stage, medical device company that has developed a disruptive peritoneal dialysis (PD) device based on revolutionary new filtration technology developed in-house.
The Device aims to be first in the world for wearable dialysis device. .
Refine business model and expand markets in the $120 billion Chronic Kidney Disease market
The current treatment modalities are disruptive to the patient’s daily routine but also impose a heavy cost burden on patients, payers, and caregivers. Presently patients are required to be plugged to a machine in a dialysis center during the day or overnight at home, requiring either travel time and a flexible work schedule or limiting their mobility.
Additionally these treatments require large amounts of water (several hundred of liters) and are power intensive, so there is also an incentive to develop new technologies which will require less energy and water. The high cost burden of in center treatment has led to a continuous shift in preference towards at home dialysis options.
VIVANCE technology runs on breakthrough sorbent-based regeneration technology that eliminates the current dependence on large volumes of dialysate and costly water treatment, while also being 20x smaller than in-center dialysis machines. These lightweight and portability features enhance a patient’s mobility which instils self confidence and psychological well being.
VIVANCE Technologies is a clinical-stage, medical device company that has developed a disruptive peritoneal dialysis (PD) device based on revolutionary new filtration technology developed in-house.

the_title
- Sector: thesector
- Investment: theinvestment
- Website: thewebsite